On June 28, Cognition Therapeutics announced top-line results of the Phase 2 SEQUEL (COG0202) study, which tested CT1812 in 16 adults with mild-to-moderate Alzheimer’s disease. Quantitative electroencephalography (qEEG) was used to assess whether four weeks of treatment with CT1812 had an impact on brain activity.
Results indicate that CT1812 may have a beneficial effect on regional brain function as well as on connectivity of brain regions.
 The distinct regions of the brain, as described to the right, are responsible for specific functions and personality attributes. Damage to these regions either by traumatic injury or disease, can impair those functions.
The distinct regions of the brain, as described to the right, are responsible for specific functions and personality attributes. Damage to these regions either by traumatic injury or disease, can impair those functions.
The hippocampus, which resides in the temporal lobe of the brain and is involved in learning and memory, is often the first to be affected by Alzheimer’s disease. This damage can make it difficult to a person with Alzheimer’s to retrieve recent memories or learn new information.
As Alzheimer’s disease progresses, not only does the brain lose connections between nerve cells, it also loses connections between brain regions. This can make it increasingly difficult to recognize a familiar person visually or navigate around a familiar location.
These changes within and between brain regions often precede noticeable worsening of symptoms. One way to measure these changes is through the use of quantitative electroencephalogram (qEEG).
 An EEG is a sensitive test that measures the electrical activity of neurons in the brain by placing a matrix of small discs (electrodes) on the scalp. Each electrode measures the summed activity of the brain region underneath. The resulting EEG readout will appear as a series of wave forms representing the activity of specific brain regions.
An EEG is a sensitive test that measures the electrical activity of neurons in the brain by placing a matrix of small discs (electrodes) on the scalp. Each electrode measures the summed activity of the brain region underneath. The resulting EEG readout will appear as a series of wave forms representing the activity of specific brain regions.
The fastest waves – those with the greatest frequency per second – are termed “gamma” (greater than 30Hz) followed by “beta” (13-30Hz), “alpha” (8-12 Hz), “theta” (4-8 Hz), and “delta” (less than 4Hz).
In cognitively normal individuals in a restful but awake state, the dominant brainwaves in the posterior (in the parietal and occipital regions) are alpha waves. These are considered to be part of the normal background rhythm of a healthy brain.
 To assess the performance of a brain region, the “relative power” of brain waves are determined. Relative power refers to the share of power among the different bands. Relative power changes in the normal aging process, with a decrease in relative alpha power, suggesting cognitive processing speed is slowing. Concurrently, slower wavelengths (theta and delta) gain in relative power as a person ages.
To assess the performance of a brain region, the “relative power” of brain waves are determined. Relative power refers to the share of power among the different bands. Relative power changes in the normal aging process, with a decrease in relative alpha power, suggesting cognitive processing speed is slowing. Concurrently, slower wavelengths (theta and delta) gain in relative power as a person ages.
Studies have shown that patients who decline from mild cognitive impairment to symptomatic Alzheimer’s disease experience an increase in relative power in the theta band, indicating a slowing overall in brain activity.
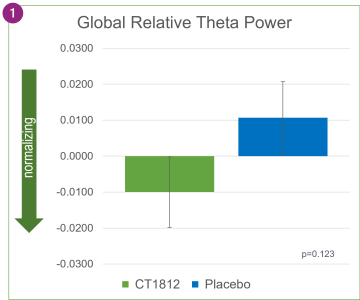
As shown in Figure 1 above, treatment with CT1812 was associated with decreases in relative theta power in specific brain regions: frontal, temporal, posterior (parietal and occipital), and central. After four weeks of treatment, the change in the central region (p=0.006) was nominally statistically significant.
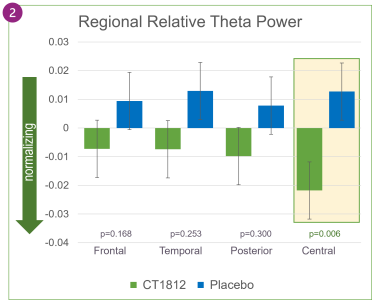
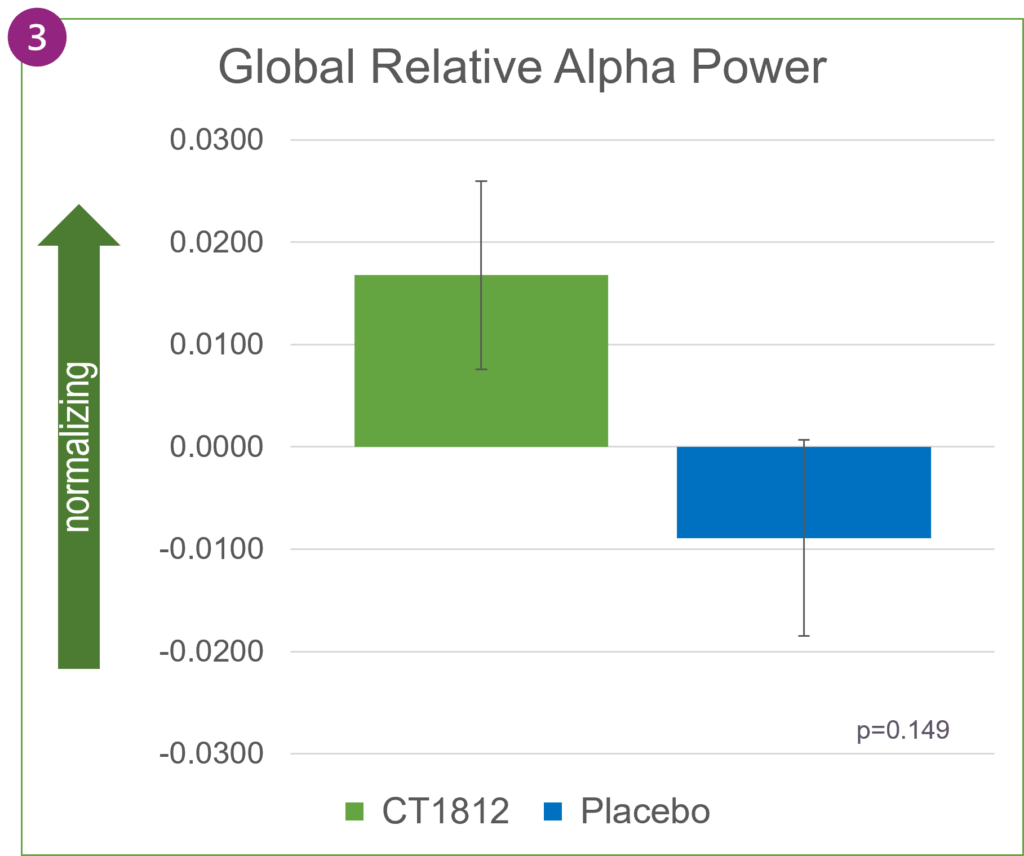
 When averaged across the entire brain, as shown in Figure 2, global relative theta power changed directionally in favor of treatment with CT1812 but was not statistically significant. Increases in relative alpha power were also observed globally in participants treated with CT1812.
When averaged across the entire brain, as shown in Figure 2, global relative theta power changed directionally in favor of treatment with CT1812 but was not statistically significant. Increases in relative alpha power were also observed globally in participants treated with CT1812.
In Alzheimer’s disease, alpha waves typically lose their dominance and are gradually replaced by slower-oscillating, lower-amplitude theta waves. In SEQUEL, the favorable trend in increasing alpha power and decreasing theta power reflects a positive impact of CT1812 on brain activity.
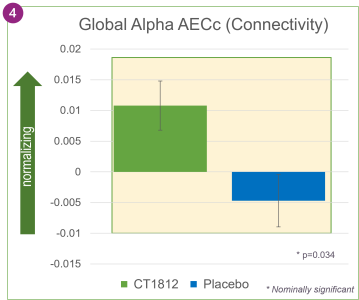
In addition, an analysis of the qEEG results showed that CT1812 treatment was associated with greater connectivity between brain regions, as measured by AECc. This improvement was nominally statistically significant with a p-value of 0.034, suggesting that the brain’s ability to communicate and exchange information between regions can be improved by CT1812.
As observed in previous studies, CT1812 was well-tolerated in the SEQUEL study with all adverse events being mild-to-moderate in severity. There were no treatment-related SAEs reported. Consistent with previous experience, there was one incident of mild elevated liver enzymes.
These results were summarized on 6/28/23 in a press release and a subsequent webcast. In addition, they were presented in a poster at CTAD 2023 by Willem de Haan, M.D., Ph.D., a neurologist and senior researcher at the Amsterdam University Medical Centers’ Alzheimer Center.
