Alzheimer’s disease is believed to be caused, in part, by the age-related buildup of proteins including amyloid beta (Aβ).
Under normal conditions, these proteins are removed from the brain before they accumulate and aggregate into more toxic forms, called oligomers. When this function is disrupted, the formation of toxic oligomers can lead to neurodegenerative disorders such as Alzheimer’s disease. These toxic oligomers bind to synapses and cause a cascade of damage resulting eventually in the loss of neurons and cognitive dysfunction.
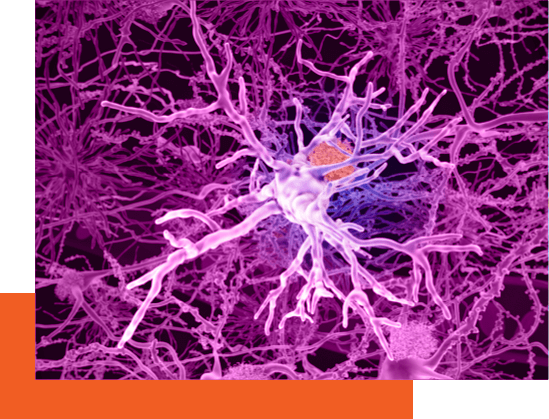
In Alzheimer’s disease, evidence suggests that Aβ oligomers are toxic to synapses in the brain. When oligomers bind to neurons, they damage synapses, which are the points of communication between neurons. Over time, the recurring damage to synapses leads to loss of neurons and cognitive decline.
Many efforts in drug development for Alzheimer’s disease have focused on removing Aβ plaques that have accumulated from the brain. We believe that preventing Aβ oligomers from binding to the synapse, where their toxic effects initiate damage, is a promising neuroprotective approach acting earlier in the amyloid cascade.
Cognition Therapeutics is developing zervimesine (also CT1812), an oral, brain-penetrant, small molecule therapeutic, which has been shown to protect neurons and synapses by preventing the binding of toxic oligomers. We believe that the results of our clinical trials demonstrate that zervimesine acts as a neuroprotective agent both by shielding neurons and synapses from oligomer binding and by preventing oligomers from attaching to synapses in the first place. Zervimesine may help mitigate the neurotoxic effects, slowing cognitive decline and progression of Alzheimer’s disease.
Zervimesine has the potential to work in synergy with recently approved monoclonal antibody treatments. Due to its unique mechanism of action and favorable safety profile, zervimesine has the potential to complement treatments that remove plaques or impact other disease mechanisms by playing the vital role of synaptic protector.
Watch our mechanism of action video to learn more. »
- Complete with results: 153 adults with mild-to-moderate Alzheimer’s disease were randomized to receive one of two doses of zervimesine or placebo.
- Supported by appx $31 million in grant awards from the National Institute on Aging (NIA) of the National Institutes of Health (NIH).
- On July 29, 2024, a webcast was held to review findings from the SHINE study. Click here for the archive.
- Cognition conducted a second call on October 30, 2024 to review findings of a pre-specified analysis of SHINE subgroups defined by their baseline plasma p-tau217 concentrations. An archive of this call and supporting materials may be found here.
- For more information, please visit clinicatrials.gov and reference NCT03507790.
- Assessed disease-modification as a function of synapse function as measured by qualitative EEG.
- Topline results showed improvements in global measures of brain activity.
- For more information, please visit clinicatrials.gov and reference NCT04735536.
- Background information on qEEG and a summary of topline results maybe found here.
- Assessed target engagement vis-a-vis displacement of Aβ oligomers.
- For more information, please visit clinicaltrials.gov and reference NCT03522129.
- Assessed disease-modification as a measure of synapse density with PET imaging.
- For more information, please visit clinicaltrials.gov and reference NCT03493282.
- Zervimesine was generally safe and tolerated in patients with mild-to-moderate Alzheimer’s disease.
- All adverse events were mild or moderate; there were no serious adverse events.
- Zervimesine achieved CSF concentrations greater than 80 percent estimated brain receptor occupancy, a level previously demonstrated in preclinical studies to be the minimum efficacious dose.
- Protein biomarkers associated with Alzheimer’s disease changed differentially in zervimesine-treated vs. placebo patients.
- For more information, please visit clinicaltrials.gov and reference NCT02907567.
START COG0203 study:
- Phase 2 study in early Alzheimer’s disease in collaboration with the Alzheimer’s Clinical Trials Consortium (ACTC).
- Target enrollment: 540
- Supported by a $81 million grant from the National Institute on Aging (NIA) of the National Institutes of Health (NIH).
- For more information, please visit start-study.org or clinicatrials.gov and reference NCT05531656.

